SIRIS Hip & Knee implant registry
Logical structure of SIRIS Hip & Knee registry
The SwissRDL documentation platform was developed to support data separation. The SwissRDL central server, located at the University of Bern, handles the software applications and the central database, along with all study definitions and anonymised clinical study data.
All personally identifiable data (demographic data on users, clinics and patients) are stored on SwissRDL module servers. No direct connection exists between the central server and the module servers.
Data validation Flexible options were developed for data sharing and access based on individual requirements. Data quality is automatically controlled by means of systematic validation of incoming data. The data is checked for completeness and plausibility. If necessary, users are requested to correct or complete data.
Data separation Incoming data is separated. Only the anonymised patient number (Pat. no.), the internal identification numbers for the user, the clinic, the department and the module are transmitted to the SwissRDL central server. Clinical data is never transmitted beyond the module server. The sole demographic data stored on the central server are the patient’s year of birth and sex. This enables epidemiological analyses to be performed on the entire data set.


Collection of the clinical data
The SIRIS Hip & Knee registry enables distinct and cross-institutional tracking of implants without violating patients’ rights to privacy. Data on implants and procedures is collected and transmitted to the registry in pseudonymised form only once patients sign a declaration of consent.
Pseudonymisation involves replacing the name or another means of identification with a pseudonym (code) to prevent identification of the patient. Thanks to the specialised architecture of the server environment and data encryption, the traceability of implants and patients is guaranteed.
In addition to patient name, type of procedure, time, surgeon and clinic, also recorded are patient characteristics (sex, age, height, weight, ASA score, Charnley score), diagnosis and technical details of the operation (surgical approach, positioning, aids) by means of a validated catalogue of questions (all available questionnaires can be found under > Downloads).
Individually adapted question catalogues are employed for hips and knees. The relevant questionnaire can be completed by the surgeon, their assistants or by the administrative office. The questions have been adapted to conform with international standards. The question catalogues are classified based on the following areas of application:
Catalogue of questions for primary operations
- Patient data (first name, last name, date of birth, sex, patient ID, etc.)
- Clinic, surgeon, date of operation
- Risk factors (ASA, body mass index)
- Principal pathology (diagnosis, previous operations)
- Procedure (date, side of body and type of procedure)
- TTechnology (conventional, minimally invasive, computer-assisted)
- Fixation of components (fixation type, cementing technique and cement)
- Implant data
Catalogue of questions for revision operations
- Pathology (reason for revision/diagnosis)
- Remaining entries as per the standard questionnaire
Swiss Orthopaedics hip and knee expert groups continuously review whether the questions are up to date and, if necessary, adapt them in conjunction with the SIRIS Scientific Advisory Board (e.g. robot-ics, surgical approaches and positioning).
Last up-date, january 2021.

Collection of the implant data
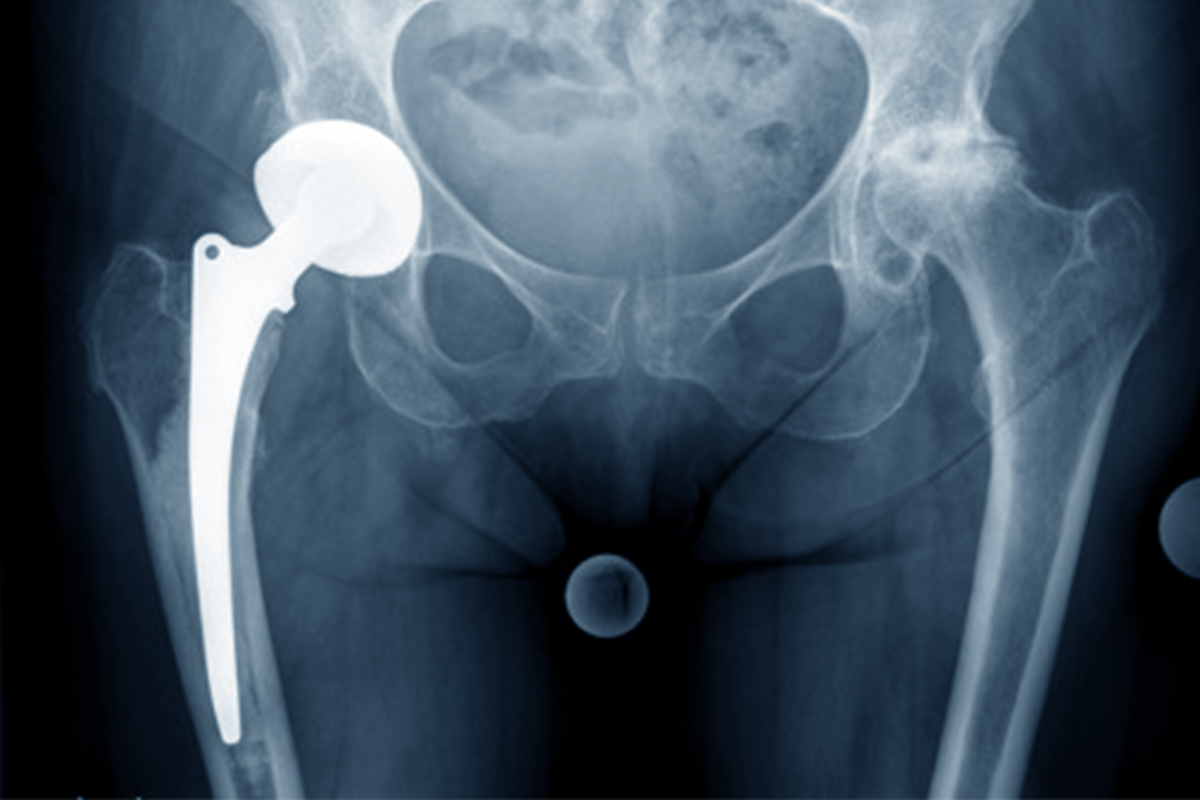
One of the primary tasks of the SIRIS registry is to reliably record which implants and cements have been used. Thanks to the implant database in the registry system and updates in the input mask, implant data acquisition quality has been significantly improved.
Implants are transmitted to the registry either via a drop down menu, by scanning using a barcode reader or via an automatic web interface.
he SwissRDL registry mask has been designed with the following measures to enhance quality.
1. To conduct a manual search, only the first section of an individual “article number” is required
2. When inputting article numbers, special characters such as “/”, “-”, “.”, etc. can be omitted.
3. Frequently used products can easily be added to a list of “favourites". The “favourites” list replaces the old “component notebook”
For new or unknown products, we request users send a photo of the sticker to implants@ispm.unibe.ch so that SwissRDL can update the implant database at the earliest opportunity. Detailed information on the input masks can be found in the manual. > LINK
Collection methods for clinics
There are several ways to keep data collection as simple as possible for the clinics.
Online entry With the help of a password, the orthopaedic surgeon can directly access their input mask in the SwissRDL registry system. The physician and/or hospital administration inputs the patient and operation data online.
Link with the hospital information system (HIS) Data is input into the hospital’s own HIS and transmitted to an allocated interface of the SwissRDL registry system. For further information regarding installation of this interface solution, please directly contact the SwissRDL registry operator team >LINK.
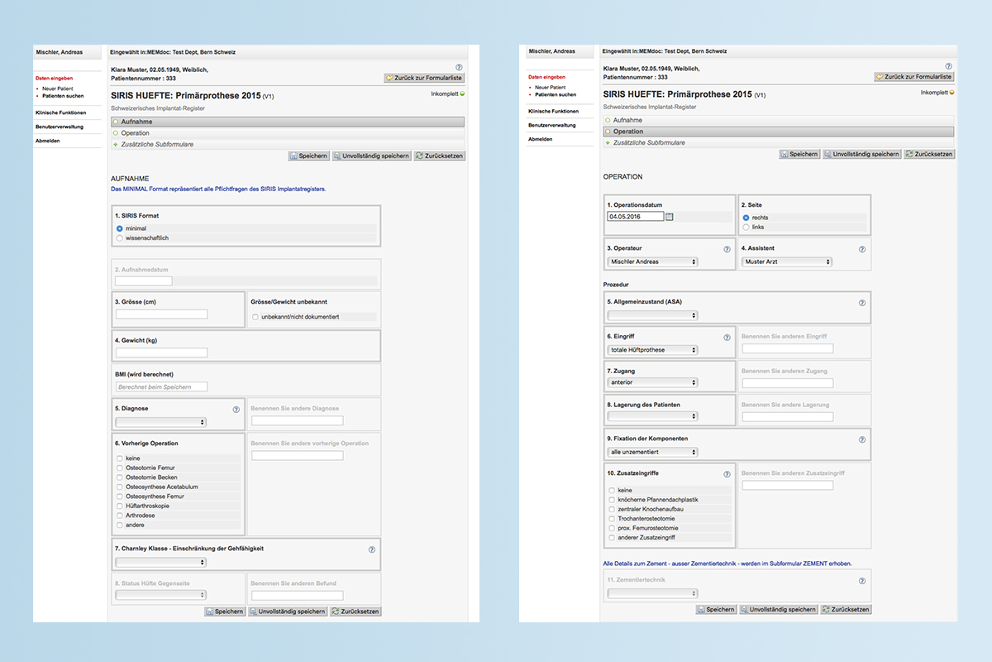
SIRIS app Input online and offline via the SIRIS app, transmission via interface directly to the SwissRDL registry system (see figure). For further information regarding installation of this app solution, please directly contact the SIRIS administrative office >LINK.
Printed forms Data entry forms are available as PDFs for each field (hip, knee, primary, revision and follow-up). Clinics are responsible for the printing of forms. The completed questionnaires should be entered into the SwissRDL registry system by hospital administrative staff. Printed forms will be discontinued in the coming years.

Data access and data processing
The SIRIS Hip & Knee implant registry is not publicly accessible. To prevent unauthorized processing of data, appropriate technical and organisational measures have been taken to guarantee the confidentiality, availability and accuracy of data.
Regulations for use A set of regulations defines which users are authorised to use the registry, the extent to which they are granted access and the fees for use. In general, the widely accepted ANQ data regulations also apply to the SIRIS implant registry.
Patient data In principle, personal data can only be viewed by attending physicians, hospitals and the institute responsible for maintaining the registry. Authorised persons are obliged to observe absolute confidentiality. The foundation may consent to further use of the data in completely anonymised form upon request. Swiss federal data protection regulations will be fully complied with. Patients can view their data at any time and, if necessary, request their deletion.
Access for orthopaedic surgeons and hospitals All orthopaedic surgeons and hospitals may view the data they have provided and compare it with aggregated anonymous data (benchmark). All decisions on further use of data and data sets are made by the Foundation Board. The Foundation Board is also obliged to issue and freely publish an annual report.
Data sovereignty Clinics retain ownership of their raw data. The foundation has ownership and holds all intellectual property rights to the database. Upon request, patients are entitled to access their information at any time and free of charge, as provided for in the Swiss Federal Act on Data Protection.
Reporting modalities of SIRIS Hip & Knee
Quarterly reports for clinics Four times a year, SwissRDL generates cumulative analyses for each clinic. Designated SIRIS administrators are responsible for distributing these within clinics. The report for the fourth quarter presents an annual overview. The reports describe the most important variables recorded in the registry for each clinic and compare them with the pooled data of the participating clinics (see figure below).
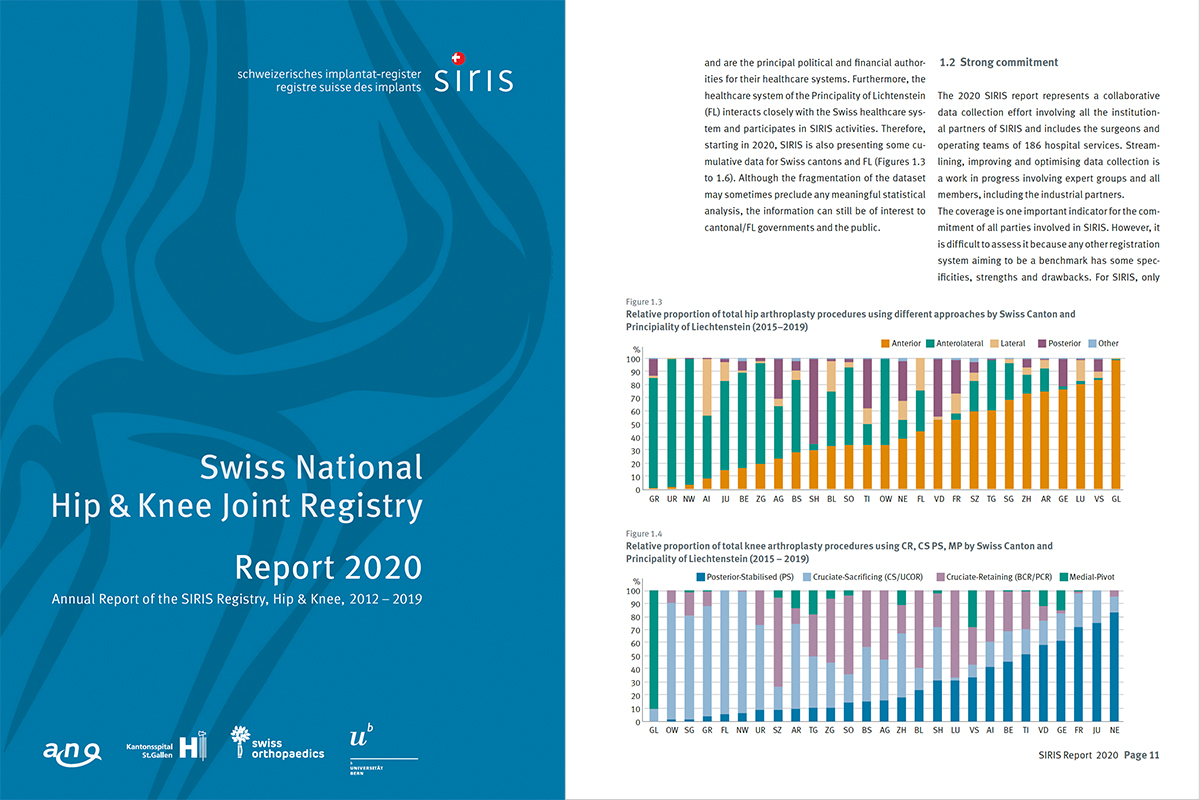
Annual Scientific Report The Annual Scientific Report is prepared by SwissRDL in collaboration with the SIRIS Scientific Advisory Board. The ANQ SIRIS Expert Group plays a consultative role (Sounding Board).> LINK to the current SIRIS Report (see picture).
National transparent hospital/clinic comparison Concurrent to the publication of the Annual Scientific Report, transparent clinic-level evaluations (revision rates) are published on the ANQ website as per a publication guideline. These reports are prepared in close cooperation between the SIRIS Scientific Advisory Board, SwissRDL, the ANQ SIRIS Expert Group and the SIRIS Foundation. The ANQ has the right of first publication.
Summary of the Annual Scientific Report The ANQ publishes an annual summary of the most important findings from the detailed Annual Scientific Report. This summary is available in the three official languages of Switzerland.> LINKSurgeon’s report In the first quarter of the year, each registering surgeon is sent a personal report on the previous year via a secure download, the content of which follows the guideline on clinical re-ports (first published: spring 2021).
Implant reports After signing a contract with the SIRIS Foundation, industrial manufacturers may request, for a fee, a standardised report on individual products or product groups from the SIRIS database. These reports provide an overview of utilisation over time, the surgical procedures and fixations employed, and the trends in revisions performed in comparison with similar implant types.
Outlier report for implants One of the principal tasks of the implant registry is to monitor “outliers” identified by statistical analyses (for a definition see the SIRIS Evaluation Guideline by means of a comprehensive review procedure. Clinics and orthopaedic surgeons who utilise these products and the companies that manufacture them are provided with individual reports by secure channels and in accordance with strict regulations. The SIRIS Foundation provides access to specialists via the SIRIS Scientific Advisory Board (extended analysis and expertise to remedy causal factors).